Malaria is regarded as the biggest killer of humans. Over 50 billion humans may have died due to malaria in the entire human history.
Even now, over 200 million annual cases of malaria are reported worldwide each year, resulting in over 600,000 deaths.

Efforts have been made to develop effective controls against the mosquito vector through the use of pesticides, but these have led to the development of pesticide-resistant mosquitoes. Similarly, the use of antiparasitic drugs has led to drug-resistance parasites. As the pesticidal and parasiticidal approaches have failed, focus has moved to vaccine development as an alternative. However, the complex parasitic life cycle has confounded efforts to develop efficacious vaccines, and consequently the FDA has not approved any malaria vaccine.
US Army seems to have opened a front against Malaria. A recent patent application discloses that the US Army has succeeded in developing an unexpected multivalent malarial vaccine that overcomes the failure and deficiencies of prior malarial vaccine strategies.
Apical Membrane Antigen-1 (AMA-1) is a protein that has an essential role in malaria merozoite invasion in host red blood cells. Initial vaccines containing AMA-1 from a single strain showed some protection; however, this protection was only observed against a strain that was homologous to the vaccine strain. The lack of protection against non-vaccine (divergent) strains, has made it difficult to produce a globally effective AMA-1 vaccine, given that hundreds if not thousands of strains are found in nature.
The patent discloses methods of stimulating, inducing, promoting, increasing, or enhancing an immune response against malaria in a subject. Specifically, the method includes administering to a subject an amount of an AMA-1 immunogenic peptide, epitope, nucleic acid, composition, antibody or combination thereof sufficient to stimulate, induce, promote, increase, or enhance an immune response against malaria in the subject. Such immune response methods provide a subject with protection against malaria infection.
The treatment of an infection can be initiated at any time during the infection. A single or multiple doses may be given to achieve a reduction in the onset, progression, severity, frequency, duration of one or more symptoms or complications associated with or caused by malaria infection. Thus, the treatment method can be practiced one or more times in an hour, day, week, month, or year.
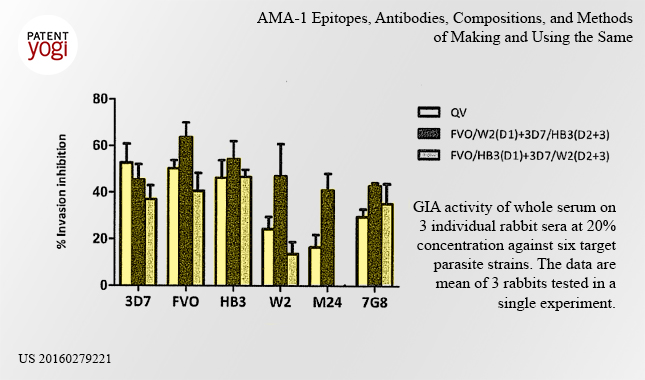
Currently, the Army is testing the vaccine in animals, such as rabbits.
Publication number: US 20160279221
Patent Title: AMA-1 Epitopes, Antibodies, Compositions, and Methods of Making and Using the Same
Publication date: 29 Sep 2016
Filing date: 11 May 2016
Inventors: Sheetij Dutta; John David Haynes; Adrian Batchelor; Michael Foley;
Original Assignee: THE GOVERMENT OF THE UNITED STATES, AS REPRESENTED BY THE SECRETARY OF THE ARMY